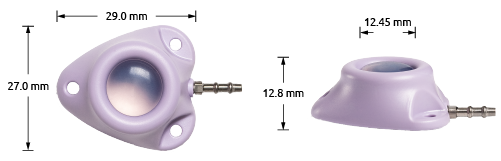
C-Port®-CT

The C-Port®-CT Port Catheter System.
Implantable venous port - CT compatible.
The C-Port®-CT, our economical plastic version of an implantable port that is also suitable for high pressure infusion.
The C-Flow® chamber of the C-Port®-CT differs from most conventional ports with a cylindrically shaped port chamber by the optimized inner chamber geometry and the baseline outlet. This results in improved flow dynamics and allows, in addition to standard applications, the performance of computed tomography with contrast medium (contrast-CT).
The lightweight, tissue-compatible plastic is CT/MRI compatible.
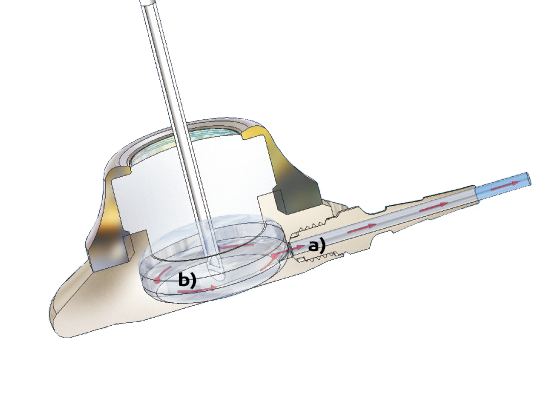
a) Clear septum and baseline outlet simplifies port venting and minimizes the risk of air embolism.
b) The C-Flow® chamber improves flow dynamics and reduces the risk of occlusion.

The C-Port®-CT is characterized by the special heart shape. This is also clearly visible in the X-ray image. Furthermore, the heart shape supports the blunt preparation of the port pocket during implantation.

The connection mechanism enables safe connection of the catheter to the port base via audible and tactile feedback. These two detection functions ensure that the connection is properly secure.

The raised edge of the C-Port®-CT makes it easy to palpate the port and locate the septum for infusion or CT application.
Specifications
Single lumen venous system for
Implantation by means of venous cutdown
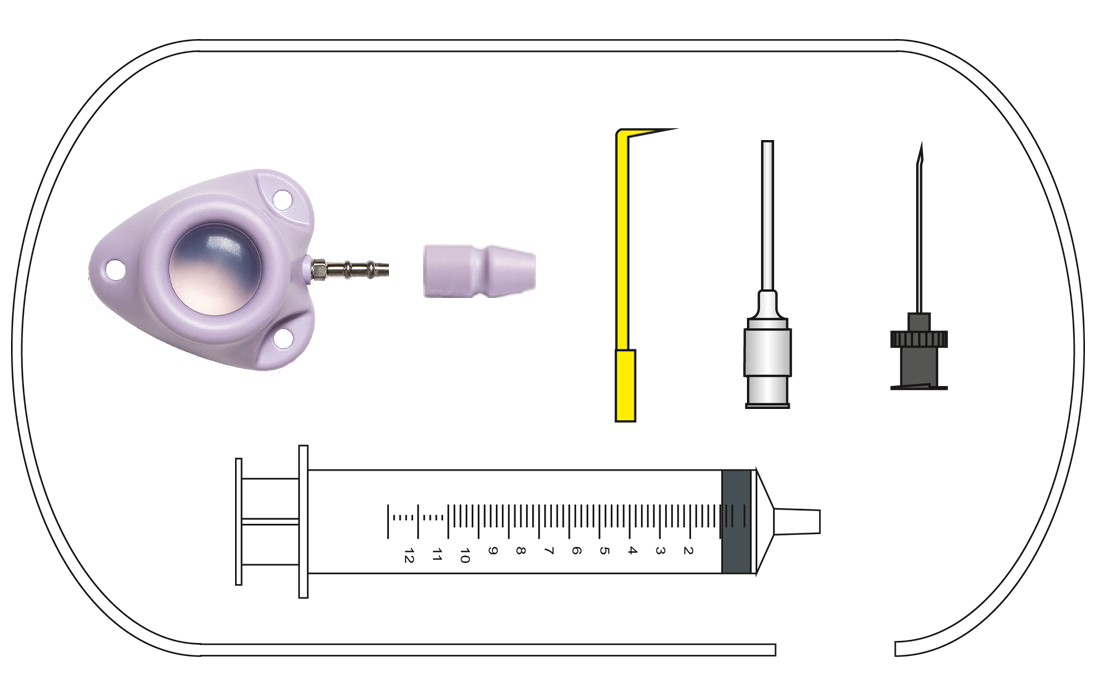
Content:
C-Port®-CT, catheter, catheter fixation,
Huber cannula straight, irrigation cannula,
vein lifter, 10 ml syringe
with percutaneous introducer set
Single lumen venous system for
Implantation by means of percutaneous technique
Content:
C-Port®-CT
Basis-System, Punktionskanüle,
Split-Schleuse mit Dilatator,
Führungsdraht mit Einführhilfe,
Tunneler
C-Port®-CT Basic system, introducer
(Seldinger) needle,
Split-Sheath with dilator,
Guide wire with insertion aid, tunneler
Ordering Information
| Catheter | Größe | O.D. | I.D. | Length | Ref. without Introducer Set |
Ref. with Introducer Set |
| Polyurethane | 6.6F | 2.20mm | 1.28mm | 70cm | CTKP-066CP | CTKP-066IP |
| Polyurethane | 7.5F | 2.50mm | 1.15mm | 50cm | CTKP-075CP | CTKP-075IP |
| Silastic®-Silicone | 8.0F | 2.67mm | 1.40mm | 50cm | CTKP-008CS | CTKP-008IS |
| Polyurethane | 9.0F | 3.00mm | 1.60mm | 50cm | CTKP-009CP | CTKP-009IP |
| Silastic®-Silicone | 9.6F | 3.18mm | 1.58mm | 50cm | CTKP-096CS | CTKP-096IS |