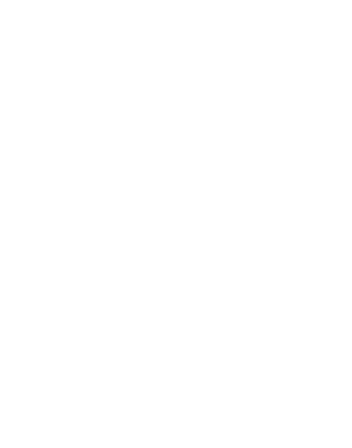
C-Port®

The C-Port® port catheter system is our economical variant of a conventional port.
The C-Port® with C-Flow® chamber differs from most conventional ports with cylindrically shaped port chambers by the optimized geometry of the inner chamber and the baseline outlet. Improved flow dynamics are ensured here.
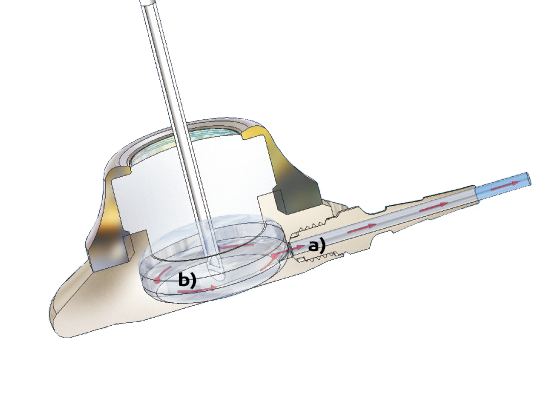
a) Clear septum and baseline outlet simplifies port venting and minimizes the risk of air embolism.
b) The C-Flow® chamber improves flow dynamics and reduces the risk of occlusion.

The connection mechanism enables safe connection of the catheter to the port base via audible and tactile feedback. These two detection functions ensure that the connection is properly secure.
Specifications
Single lumen venous system for
Implantation by means of venous cutdown
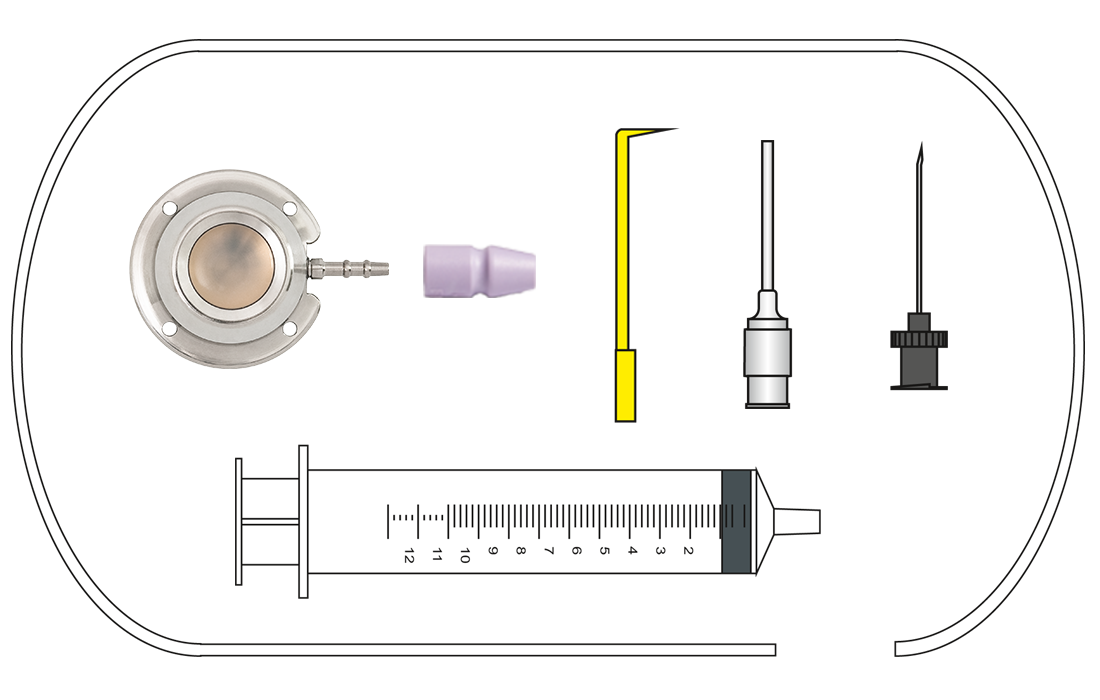
Content:
C-Port®, catheter, catheter fixation,
Huber cannula straight, irrigation cannula,
vein lifter, 10 ml syringe
with percutaneous introducer set
Single lumen venous system for
Implantation by means of percutaneous technique
Content:
C-Port® Basic system, introducer
(Seldinger) needle,
Split-Sheath with dilator,
Guide wire with insertion aid, tunneler
Ordering Information
| Catheter | Größe | O.D. | I.D. | Length | Ref. without Introducer Set |
Ref. with Introducer Set |
| Polyurethane | 6.6F | 2.20mm | 1.28mm | 70cm | SPB-066CP | SPB-066IP |
| Polyurethane | 7.5F | 2.50mm | 1.15mm | 50cm | SPB-075CP | SPB-075IP |
| Silastic®-Silicone | 8.0F | 2.67mm | 1.40mm | 50cm | SPB-008CS | SPB-008IS |
| Polyurethane | 9.0F | 3.00mm | 1.60mm | 50cm | SPB-009CP | SPB-009IP |
| Silastic®-Silicone | 9.6F | 3.18mm | 1.58mm | 50cm | SPB-096CS | SPB-096IS |